Explanation of symbols and terms used in the labeling of devices manufactured by HTL-STREFA S.A.
References obtained from ISO 15223-1:2021 Medical devices — Symbols to be used with information to be supplied by the manufacturer
Symbol
Title of symbol
Description of symbol
Reference no. as per ISO 15223:2021

Manufacturer
Indicates the medical device manufacturer
Reference no. as per ISO 15223:2021
5.1.1

Date of manufacture
Indicates the date when the medical device was manufactured.
Reference no. as per ISO 15223:2021
5.1.3

Authorized representative in the European Community/ European Union
Indicates the authorized representative in the European Community/European Union.
Reference no. as per ISO 15223:2021
5.1.2

Authorised representative for Switzerland
Indicates the authorised representative in Switzerland.
Reference no. as per ISO 15223:2021
5.1.2
Importer
Indicates the entity importing the medical device into the locale.
Reference no. as per ISO 15223:2021
5.1.8
Distributor
Indicates the entity distributing the medical device into the locale.
Reference no. as per ISO 15223:2021
5.1.9

Use-by date
Indicates the date after which the medical device is not to be used.
Reference no. as per ISO 15223:2021
5.1.4

Lot number
Indicates the manufacturer’s batch code so that the batch or lot can be identified.
Reference no. as per ISO 15223:2021
5.1.5

Catalog number
Indicates the manufacturer’s catalogue number so that the medical device can be identified.
Reference no. as per ISO 15223:2021
5.1.6

Unique device identifier
Indicates a carrier that contains unique device
Identifier information.
Reference no. as per ISO 15223:2021
5.7.10

Medical device
Indicates the item is a medical device.
Reference no. as per ISO 15223:2021
5.7.7

Sterilized using irradiation
Indicates a medical device that has been sterilized using irradiation.
Reference no. as per ISO 15223:2021
5.2.4

Sterilized using ethylene oxide
Indicates a medical device that has been sterilized using ethylene oxide.
Reference no. as per ISO 15223:2021
5.2.3
Single sterile barrier system
Indicates a single sterile barrier system.
Reference no. as per ISO 15223:2021
5.2.11

Single sterile barrier system with protective Packaging outside
Indicates a single sterile barrier system with protective packaging outside.
Reference no. as per ISO 15223:2021
5.2.14

Sterilized using irradiation / Single sterile barrier system with protective packaging outside
Indicates a medical device that has been sterilized using irradiation/Indicates a single sterile barrier system with protective packaging outside.
Reference no. as per ISO 15223:2021
5.2.4 and 5.2.14

Sterilized using ethylene oxide /Single sterile barrier system with protective packaging outside
Indicates a medical device that has been sterilized using ethylene oxide/Indicates a single sterile barrier system with protective packaging outside.
Reference no. as per ISO 15223:2021
5.2.3 and 5.2.14
Do not resterilize
Indicates a medical device that is not to be resterilized.
Reference no. as per ISO 15223:2021
5.2.6

Do not use if package is damaged
Indicates a medical device that should not be used if the package has been damaged or opened.
Reference no. as per ISO 15223:2021
5.2.8
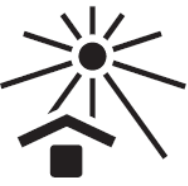
Keep away from sunlight
Indicates a medical device that needs protection from light sources.
Reference no. as per ISO 15223:2021
5.3.2

Keep dry
Indicates a medical device that needs to be protected from moisture.
Reference no. as per ISO 15223:2021
5.3.4
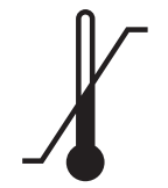
Temperature limit
Indicates the temperature limits to which the medical device can be safely exposed.
Reference no. as per ISO 15223:2021
5.3.7
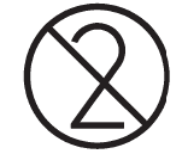
Single use / Do not re-use
Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure.
Reference no. as per ISO 15223:2021
5.4.2
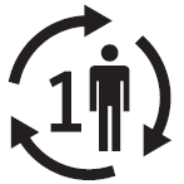
Single patient multiple use
Indicates a medical device that may be used multiple times (multiple procedures) on a single patient.
Reference no. as per ISO 15223:2021
5.4.12

Consult instructions for use
Indicates the need for the user to consult the instructions for use.
Reference no. as per ISO 15223:2021
5.4.3

Caution
Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself.
Reference no. as per ISO 15223:2021
5.4.4

Non-pyrogenic
Indicates a medical device that is non-pyrogenic.
Reference no. as per ISO 15223:2021
5.6.3
Explanation of other symbols:
Symbol
Title of symbol
Description of symbol

CE mark
Indicates that a device is in conformity with the Medical Device Regulation (EU) 2017/745 or Medical Device Directive 93/42/EEC.
Prescription use only
Indicates that a device is restricted to use by or on the order of a physician (for USA market only).

Recyclable packaging material
Indicates that packaging material is part of a recovery or recycling process.

Pack: PAP21
Non-corrugated fibreboard.

Leaflet: PAP22
Paper.
Explanation of other terms:
Term
Description of symbol
Non-toxic
Meets ISO 10993-11 (Acute Systemic Toxicity, Subacute/Subchronic Toxicity).
Not made with natural rubber latex
Natural rubber latex was not used as material in the manufacture of the device.


