HTL-STREFA S.A., a company of MTD group. We are one of the global leaders in the production of medical devices, particularly: safety lancets, personal lancets, pen needles, and lancing devices.
Our products are used by healthcare professionals for capillary blood collection in diagnostic tests conducted in hospitals, clinics, and laboratories, as well as by diabetics for daily blood glucose monitoring and subcutaneous drug administration, including insulin.

Mission
Our mission
Thanks to modern production processes and 25 years of presence in the international market, we create solutions that meet the highest quality standards. Our products are designed with the safety and comfort of users in mind – from medical professionals to patients. We focus on strategic cooperation with global leaders in the medical industry, providing them with solutions tailored to their needs. At the same time, we are developing our own brand, offering products that reflect our commitment to innovation and quality.


Close collaboration with clients and end users of our products allows us to continuously improve and adapt our offerings to the evolving needs of the market and consumers. We believe that high-quality medical devices have a real impact on people’s health and lives. Our mission is not only production, but also providing everyday support to those who care for the health and lives of others, as well as those who are living with diabetes.
For patients’ convenience, the needles produced by HTL-STREFA are compatible with most injection pens available on the market.
History
HTL-STREFA's history
Quality standards
Regulatory affairs
and quality standards
Since its very foundation, HTL-STREFA has maintained and improved a quality management system. Our quality management system complies with the EN ISO 13485:2016 standard requirements.
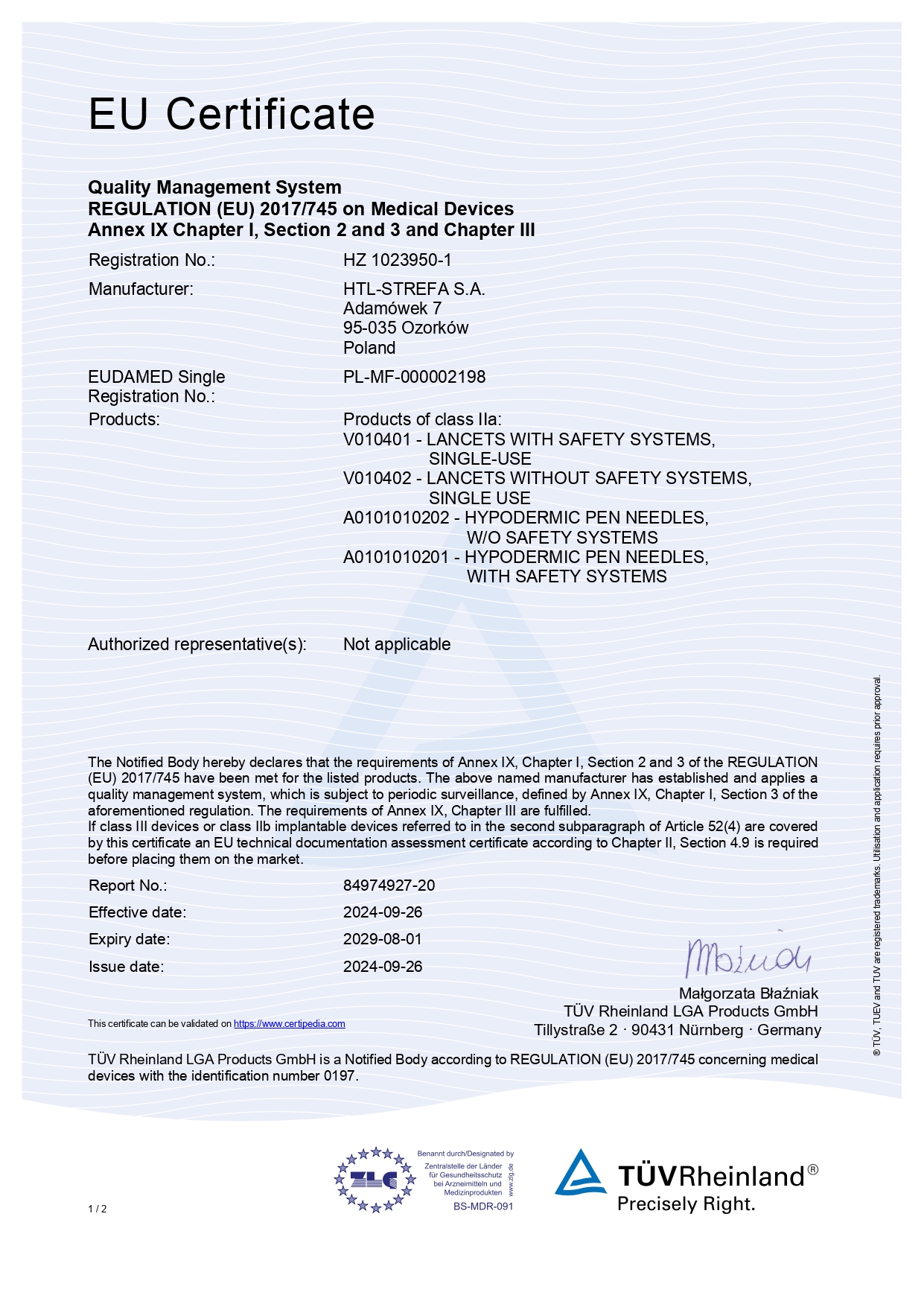
All HTL-STREFA products bear the CE marking, which proves compliance with the requirements for medical devices applicable in the European Union specified in Directive 93/42/EWG and Regulations (EU) 2017/745, (EU) 2023/607 and relevant regulations.
The Notified Body involved in the conformity assessment process of our products is TÜV Rheinland LGA Products GmbH.
Since January 2019, HTL-STREFA holds Medical Device Single Audit Program Certificate (MDSAP), confirming compliance with ISO 13485:2016, and the regulatory requirements of five jurisdictions: Australia, Brazil, Canada, Japan, and the United States.
HTL-STREFA products are sold worldwide, primarily in Europe, the USA, Canada, and South American countries, including Brazil, Mexico, China, Japan, Australia, as well as in the Middle East and Asia. We hold registrations and marketing authorizations issued by competent authorities and government agencies, as well as certificates of conformity issued by accredited notified bodies, allowing us to place the medical devices on the market, among others:
- TÜV Rheinland LGA Products GmbH. (European Union)
- FDA in the Unites States
- TGA in Australia
- Anvisa in Brazil
- HC in Canada
- MHLW in Japan
- MFDS in Korea
- COFEPRIS in Mexico
- UKRMEDCERT in Ukraine
- SFDA in Saudi Arabia
- CFDA in China
Certificates of HTL-STREFA

Manufacturing and production control
Manufacturing and production control
Currently, in Poland, we have two modern production plants – in Ozorków and Łęczyca (Łódź Special Economic Zone) – as well as a tool shop in Bydgoszcz, which allows us to build new injection molds in-house, providing the advantage of full control over the manufacturing process.
In all our production facilities we maintain complete control over the quality and design for all aspects of each product. Our team of dedicated engineers provides constant quality improvements to our products and processes, while also developing new and innovative solutions.
Ethics
Ethics
We believe that business success goes hand in hand with integrity and care for the environment. Business ethics standards are the foundation of our daily activities, both today and in the future. We operate transparently and in compliance with the law, building trust among partners, customers, and the communities we work with. We focus on the development of our business while taking into account values such as social, ethical, and environmental responsibility.