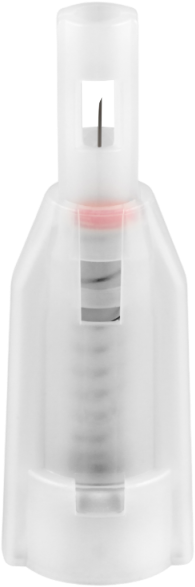
DropSafe safety pen needles are sterile, single use safety needles intended for use with pen injector devices for the injection of drugs by healthcare professional users and lay users.
Safety pen needle is a medical device. Use it according to the instructions for use and label.
“Over 80% of needlestick injuries can be prevented with the use of a safer needle devices, which, in conjunction with worker education and work practice controls, can reduce injuries by over 90%!”1 DropSafe safety pen needles have special features preventing sharps injury incidents. Before an injection needle is safely covered behind the shields, and after use is automatically locked which reduces the risk of an exposure incident and prevents a sharp injury.
Key features

Safety2
- Safety before and after injection – needle safely contained behind shield, preventing accidental exposure.
- Lock-out confirmation – red stripe appears when the needle is used and locked out.
Comfort
- Special and unique „in-house” lubrication method – Droplicon™ designed for smooth injection.4
- Hidden needle: the needle remains hidden from the view, which may increase patient comfort.4
Ease of use
- DropSafe safety pen needles work with most pen injectors available on the market.5
- Just screw it on the pen, inject and dispose.
Accuracy
- Needle viewing window for easy confirmation of drug flow (priming).6
- Thin wall (31G) may allow an easier and faster injection of the drug.3
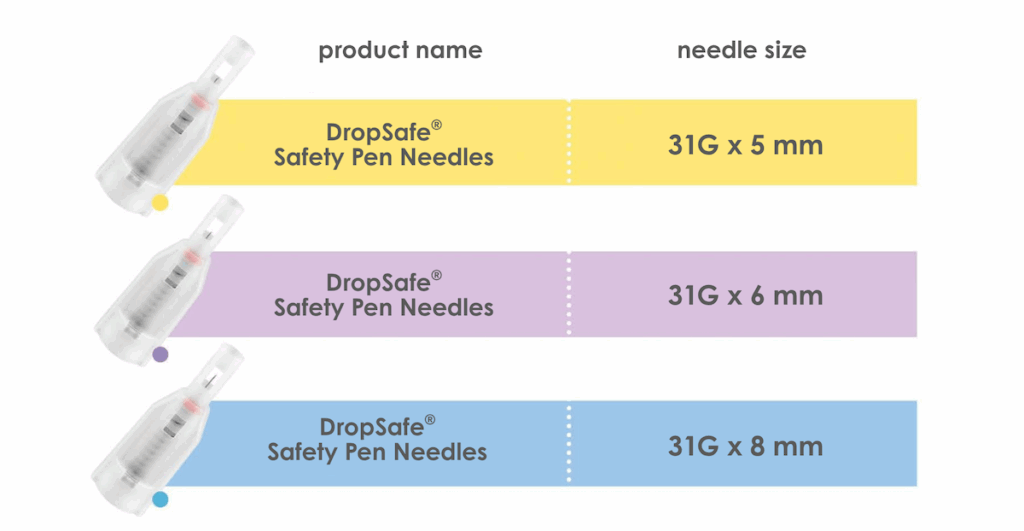
Available in three versions
DropSafe safety pen needles are available in three versions:
Compatibility
DropSafe safety pen needles are compatible with most pen injectors available on the market. See the compatibility statement for more information.
How to use DropSafe
safety pen needles:
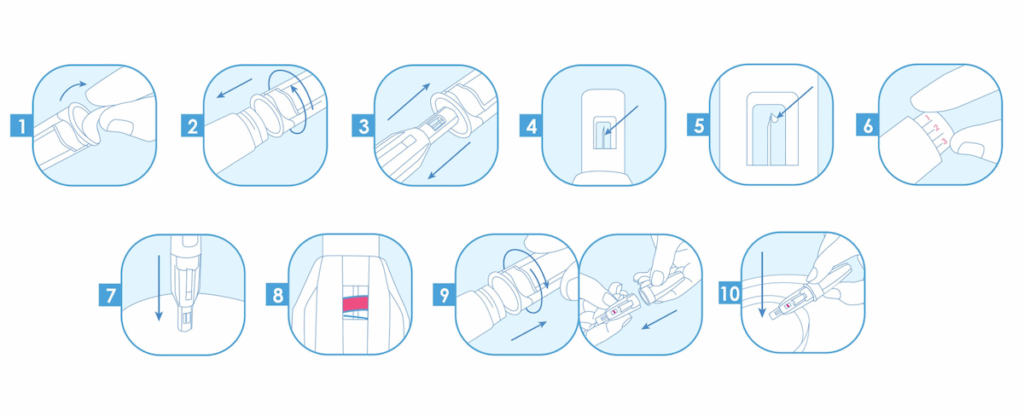
See full instruction for use and instruction video:
Product brochure
The information above is also available as a product brochure in a PDF version.
Sources:
- Światowa Organizacja Zdrowia. https://www.who.int/occupational_health/activities/1 anaism.pdf (data wydruku 12.04.2019).
- HTL-STREFA S.A. Dane w dokumentacji. RAPORT KOŃCOWY — SYMULOWANE TESTY KLINICZNE IGŁY DO NAKŁUWACZY BEZPIECZNYCH DROPSAFE. Wersja 1.0, 17 stycznia 2017 r.
- Siegmund T., Blankenfeld H., Schumm-Draeger PM. Porównanie użyteczności i preferencji pacjentów dotyczących igieł do wstrzykiwaczy insulinowych produkowanych różnymi technikami: Porównanie igieł „cienkościennych” z igłami „o zwykłej grubości”: badanie otwarte. Diabetes Technology & Therapeutics, Vol. 11, nr
- Tandon N, Kalra S, Balhara YP, Baruah MP. Chadha M, Chandalia HB i in. Forum ekspertów ds. techniki iniekcji i zaleceń terapeutycznych, Indie: Indyjskie zalecenia dotyczące najlepszych praktyk w zakresie techniki podawania insuliny. Indian J Endocr Metab 2017;21: 600-17.
- HTL-STREFA S.A. Dane w dokumentacji. Zgodność typu 820 z urządzeniami nakłuwającymi.
- HTL-STREFA S.A. Dane w dokumentacji. BADANIE JAKOŚCIOWE DOTYCZĄCE IGIEŁ DO NAKŁUWACZY BEZPIECZNYCH — WYWIADY GRUP FOKUSOWYCH Z PIELĘGNIARKAMI W HISZPANII, PMR. 20 sierpnia 2018 r.